Friday, November 5, 2021
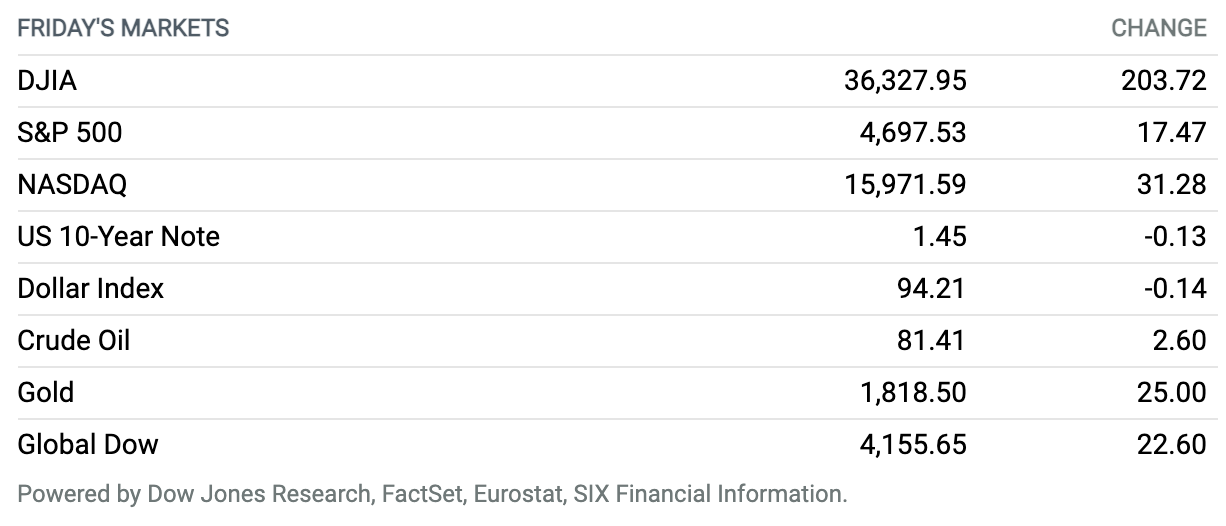
Major Stock Indexes End at Records After Strong Jobs Report. Stocks ended higher Friday, with the Dow Jones Industrial Average, S&P 500, and Nasdaq Composite all closing at new highs after a stronger-than-expected October jobs report. The Dow Jones Industrial Average rose 204 points, or 0.6%, to close near 36,328. The S&P 500 gained around 17 points, or 0.4%, to finish near 4698, while the Nasdaq Composite edged up 31 points, or 0.2%, ending near 15,972. The S&P 500 and Nasdaq each booked a seventh-straight record close. The Dow rose 1.4% for the week, while the S&P 500 advanced 2%, and the Nasdaq gained 3.1%.
The Jobs News Was Better Than Expected as Hiring Picks Up
Employers added 531,000 workers to their payrolls in October, a sign the labor shortage is improving but not over.
Economists polled by FactSet anticipated a nonfarm payrolls increase of 412,500 in October. The improved result follows two months of paltry increases, which were revised higher Friday by a combined 235,000.
Still, the labor-force participation rate remained unchanged at a low 61.6%. Economists had been hoping for a small increase, counting on more workers returning as Covid-19 infections fell and kids returned to in-person school after a year of remote learning kept about 2 million parents home.
Continue reading
White House Sets January Deadline for Covid-19 Vaccine Mandates
The White House announced details of sweeping Covid-19 vaccination mandates on Thursday, setting a deadline for many of the nation’s workers to be fully vaccinated against the virus by Jan. 4, 2022.
All U.S. workers employed by companies with more than 100 employees will need to be fully vaccinated by the deadline, or undergo weekly testing. Workers at all health-care facilities in the U.S. participating in Medicare or Medicaid—virtually every health-care facility in the nation—will also need to be vaccinated by that date, with no option to test weekly instead.
Continue reading
Pfizer’s Covid-19 Antiviral Shows Stunning Efficacy
Pfizer said its Covid-19 pill cut the risk of hospitalization or death by 89% in a trial of high-risk, nonhospitalized adults, besting by a substantial margin the efficacy of Merck‘s highly touted Covid-19 pill molnupiravir.
The results are scrambling expectations about the future of the Covid-19 oral antiviral market, which experts expect to be worth tens of billions of dollars in annual sales. Merck had been expected to be the market leader, and molnupiravir received its first authorization from U.K. health authorities earlier this week. It is set to be considered by an FDA advisory committee at the end of this month.
Continue reading
Airbnb, Expedia Earnings Signal That Travel Is Back
Expedia and Airbnb reported earnings this week and provided further signs that the travel rebound is gathering pace.
Expedia reported revenue of $2.96 billion in the third quarter, up 97% from the previous year and ahead of the consensus expectation of $2.73 billion. Airbnb said it had its “strongest quarter ever” as the travel rebound, which began earlier this year, accelerated in the third quarter.
Continue reading
Kids’ Vaccination Campaign Is Starting in U.S., With 28 Million Eligible
A campaign to vaccinate 28 million children in the U.S. against Covid-19 is set to be fully operational by next week after the Centers for Disease Control and Prevention signed off late Tuesday night on a sweeping recommendation for Pfizer‘s vaccine for children ages five to 11.
Health-care providers can begin administering the Pfizer and BioNTech vaccine to children in the age group as early as Wednesday, but the White House and the CDC said the campaign would take days to become fully operational.
Continue reading
Moderna’s Sales Miss Forecasts by 20%. Pfizer Is Taking the U.S. Vaccine Market.
Sales of Moderna’s Covid-19 vaccine fell short of estimates by more than $1 billion. The company reported total revenue of $5 billion for the third quarter of the year, 20% short of the consensus estimate of $6.2 billion. Spikevax, Moderna‘s Covid-19 vaccine, is Moderna’s only product.
The report comes two days after Pfizer said it had sold $13 billion of its Covid-19 vaccine in the third quarter, blowing past the consensus estimate of $10.9 billion.
Continue reading
Ride-Sharing Companies Buoyed by Drivers Returning to Work
Lyft posted solid third-quarter financial results this week, the company’s second straight quarter of profitability as measured by adjusted Ebitda, or earnings before interest, taxes, depreciation and amortization.
The company also offered upbeat commentary about the population of drivers. Just a few months ago, both Lyft and rival Uber Technologies were shelling out millions in drivers’ incentives in an attempt to lure back drivers who had walked away from their cars during the pandemic. Now Lyft is seeing improved driver supply, and the company expects to scale back driver incentives in the fourth quarter.
Continue reading
Retail Benefits From Growth in Services Sector, but Supply Chains Remain Stressed
Economic activity in the services sector grew at a record pace in October as the U.S. economy expanded amid a decrease in coronavirus cases and heightened consumer demand.
An index measuring economic activity in the sector jumped to an all-time high of nearly 67 in October, the Institute for Supply Management said Wednesday. The figure was up from September’s reading of 61.9, and higher than consensus expectations of 62.
Continue reading
Kroger Says News That It Will Accept Bitcoin Cash Is ‘Fraudulent’ and ‘Unfounded’
Kroger said a press release announcing the grocery retailer would accept Bitcoin Cash was “fraudulent.”
“This morning a press release was fraudulently issued claiming to be The Kroger Co. that falsely stated the organization will begin to accept Bitcoin Cash (BHC),” the company said in an emailed statement. “This communication was fraudulent and is unfounded and should be disregarded.”
Continue reading
Emergent BioSolutions Loses Key Government Contract
Emergent BioSolutions has lost a key U.S. government contract, announcing that its years-long partnership with the U.S. Department of Health and Human Services for involvement in the Center for Innovation in Advanced Development and Manufacturing (CIADM) program had been terminated.
The pandemic preparedness program included the order to reserve and expand manufacturing capacity for third-party Covid-19 vaccine and therapeutic candidates.
Continue reading
Disclosure – All investment carries risk, and we cannot guarantee performance or results. Past performance does not guarantee future results. GIA does not earn any compensation from any of the non-GIA links provided in these resources. The market insights, podcast, blogs, book recommendations, self improvement thoughts, food recipes and activities are based on our perspectives and experience, and may not apply to your unique situation or be appropriate for your health and wellness. We are not aware of any conflicts of interest relating to any testimonials or endorsements. Please contact us for any questions relating to the content above, or to discuss how we can support you in your specific situation, and help you to reach your financial and personal goals.










